Voltage-gated sodium currents influence firing patterns of vestibular afferents
The vestibular inner ear transmits head-motion information to the brain via two populations of vestibular ganglion neurons (VGN), which differ in the regularity of intervals between spikes (action potentials, AP). Regular and irregular timing appear to represent different encoding strategies: rate encoding and precise temporal encoding, respectively, optimized for different ranges of sensory information (DC (0) – several Hz vs. 5 – 20 Hz) (Jamali et al. 2016)). In vivo, regularity differences between afferent populations may arise through differences in synaptic inputs and intrinsic ion channel expression (Smith and Goldberg 1986) as well as dendritic arbors. Of particular interest are ion channels that are open at resting potential, which set the conditions for spontaneous spiking. Previous work has focused on the influence of low-voltage-activated K (KLV) channels (Iwasaki et al. 2008, Kalluri et al. 2010) and HCN channels (Horwitz et al. 2014, Ventura and Kalluri 2019) on spike regularity: KLV channels increase irregularity, while the effect of HCN channels is context dependent.
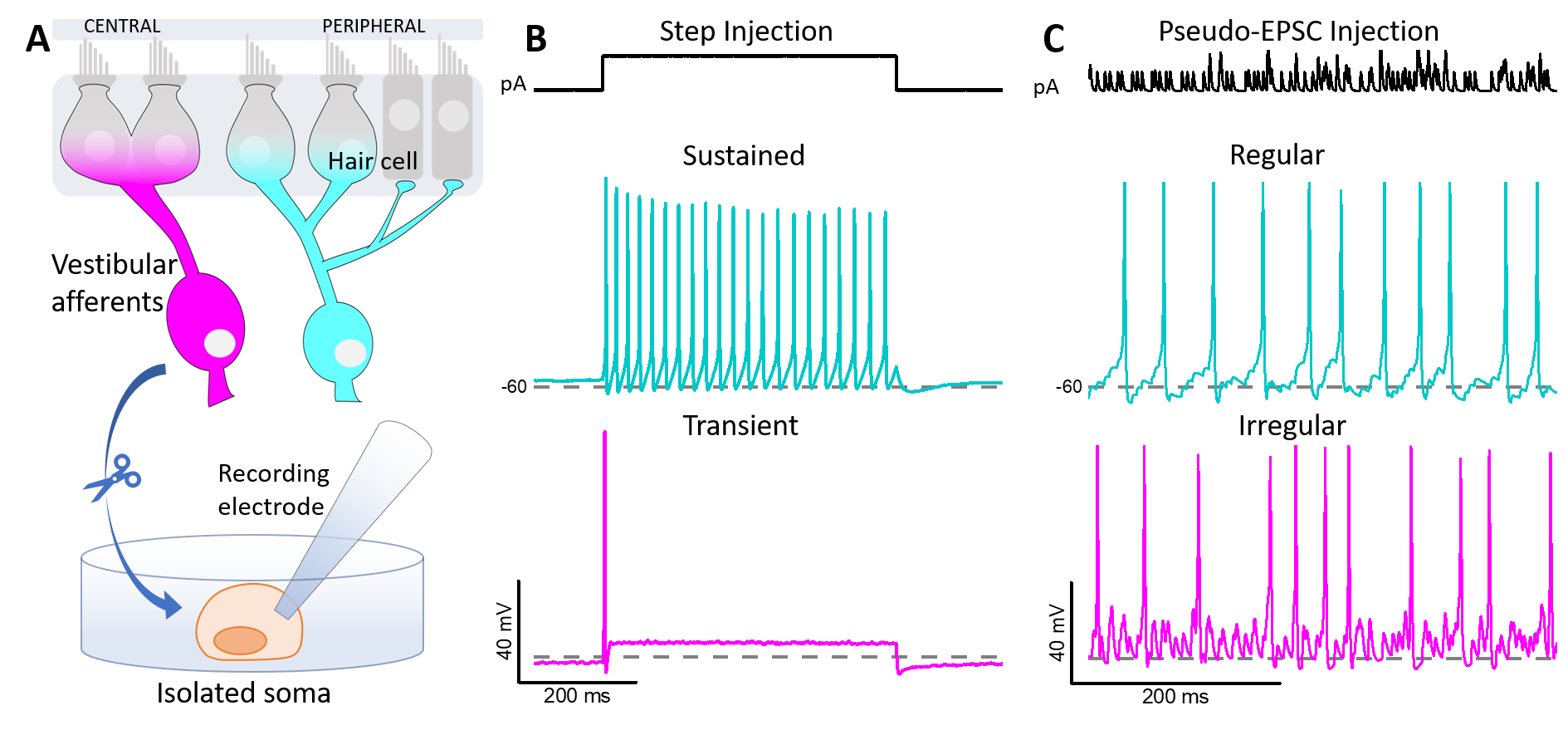
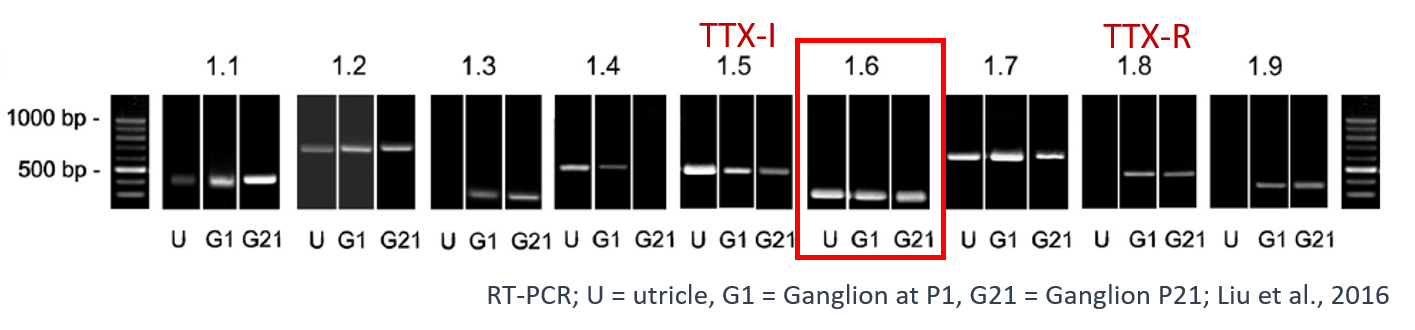
We are now exploring the roles of voltage-gated sodium (NaV) currents, which drive the upstroke of the AP, in VGN spike timing. Expression for all 9 NaV channel pore-forming (α) subunits (NaV1.1-1.9) has been detected in vestibular ganglia, and different VGN have different complements of transient (inactivating) NaV currents (Liu et al. 2016): slow and positively activating TTX-resistant currents (carried by NaV1.8); fast and negatively activating TTX-insensitive currents (NaV1.5); and 2 kinds of TTX-sensitive currents with intermediate voltage dependence and multiple candidate molecular identities, including NaV1.6 (Liu et al. 2016).
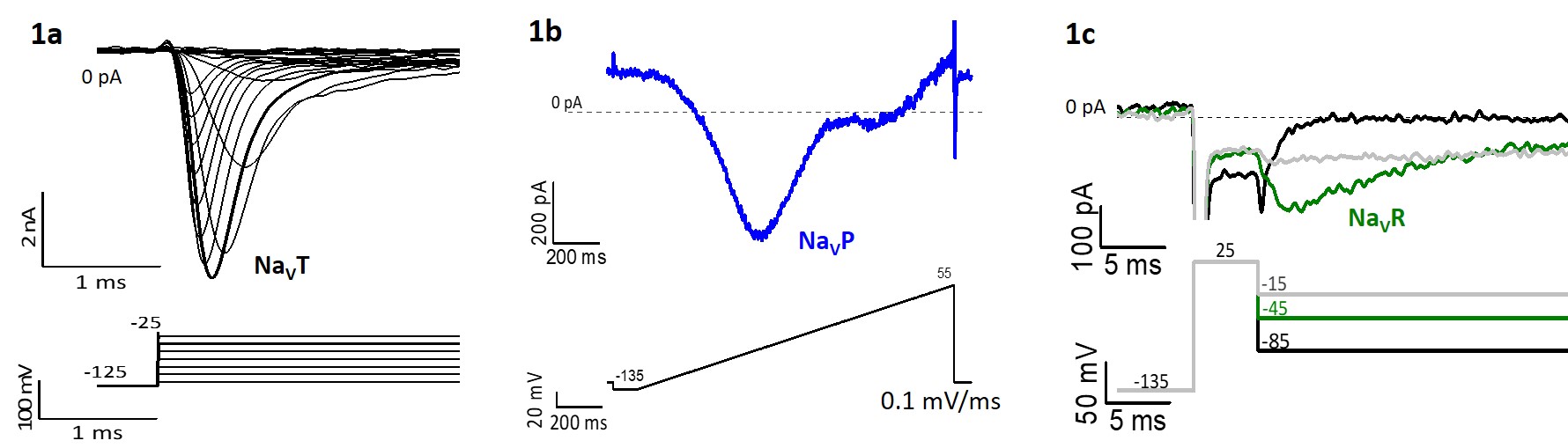
TTX-sensitive NaV currents through a given NaV1.x channel can also take different forms. In addition to large transient currents (I-NaVT; Fig. 1a), there can be small persistent currents (I-NaVP; Fig 1b) through channels that do not inactivate, and/or resurgent current (I-NaVR; Fig 1c) as channels unblock during repolarizations (e.g., on the downstroke of a spike). NaVP and NaVR currents have the potential to enhance spike rate and regularity by making more NaV channels available after a spike.
**Literature Cited**
- Horwitz GC, Risner-Janiczek JR, and Holt JR. Mechanotransduction and hyperpolarization-activated currents contribute to spontaneous activity in mouse vestibular ganglion neurons. J Gen Physiol 143: 481-497, 2014.
- Iwasaki S, Chihara Y, Komuta Y, Ito K, and Sahara Y. Low-voltage-activated potassium channels underlie the regulation of intrinsic firing properties of rat vestibular ganglion cells. J Neurophysiol 100: 2192-2204, 2008.
- Jamali M, Chacron MJ, and Cullen KE. Self-motion evokes precise spike timing in the primate vestibular system. Nat Commun 7: 13229, 2016.
- Kalluri R, Xue J, and Eatock RA. Ion channels set spike timing regularity of mammalian vestibular afferent neurons. J Neurophysiol 104:2034-51, 2010.
- Liu XP, Wooltorton JR, Gaboyard-Niay S, Yang FC, Lysakowski A, and Eatock RA. Sodium channel diversity in the vestibular ganglion: NaV1.5, NaV1.8, and tetrodotoxin-sensitive currents. J Neurophysiol 115: 2536-2555, 2016.
- Lysakowski A, Gaboyard-Niay S, Calin-Jageman I, Chatlani S, Price SD, and Eatock RA. Molecular microdomains in a sensory terminal, the vestibular calyx ending. J Neurosci 31: 10101-14, 2011.
- Smith CE, and Goldberg JM. A stochastic afterhyperpolarization model of repetitive activity in vestibular afferents. Biol Cybern 54: 41-51, 1986.
- Ventura CM, and Kalluri R. Enhanced activation of HCN channels reduces excitability and spike-timing regularity in maturing vestibular afferent neurons. J Neurosci 39: 2860-76, 2019.
